Beyond Normal Bonding
Purpose
Recently, while learning about Natural Bond Orbitals (to be used to analyze the bonding in a newly synthesized, unique organometallic species) I ran across a very interesting set of results published by Weinhold and Landers1
In High School, College and Graduate School, Chemistry students learn that molecules are composed of atoms which are bonded one to another. In most cases there is one bond between atoms. In other cases, there are two or even in some cases, three. Here we examine those orbitals and a more exotic case where there are up to 5 bonds between atoms.
Method
All the Natural Bond Orbital graphics and VRML models used on this page were derived from Natural Bond Orbital Analysis (v5)2 of Hartree-Fock STO-3G5 calculations using pcGAMESS3 (ethane, ethene, ethyne) and B3LYP Density Functional, Hay & Wadt ECP5 calculations using Gaussian 98W4 (H2W2).
Results
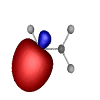
In common hydrocarbons like ethane, ethene (ethylene), and ethyne (acetylene) the atoms are connected by bond orbitals like these (click on any image for a click the appropriate link to download a 3D or Rendered file):
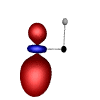
C-H Bond
C-C Bonds
These are the usual cases one encounters when learning and using chemistry.
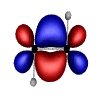
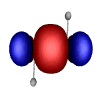
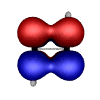
However, in exploring the theory and application of Natural Bond Orbitals to systems containing transition metals, Weinhold and Landis discussed the case of tungsten hydride (H2W2). In this case, the tungsten (W) atoms are quintuply bonded (i.e. there are 5 bonds between the tungsten atoms). These unusual bond orbitals look like this:
A very interesting contrast to the usual bonding we chemists normally consider in our work.
References:
[1] Introduction to Natural Bond Orbitals, NBO 5.0 Program, and Recent Extensions of Localized Bonding Concepts. F. Weinhold and C. R. Landis, Chem. Ed.: Res. & Pract. Eur. (CERAPIE; special “Structural Concepts” issue) 2, 91-104 (2001).
[3] Alex A. Granovsky, www http://classic.chem.msu.su/gran/gamess/index.html
[4] Gaussian 98, Revision A.7,
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria,
M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr.,
R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam,
A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi,
V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo,
S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui,
K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari,
J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu,
A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin,
D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara,
C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen,
M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon,
E. S. Replogle, and J. A. Pople,[5] Basis sets and ECP parameters were obtained from the Extensible Computational Chemistry Environment Basis Set Database, as developed and distributed by the Molecular Science Computing Facility, Environmental and Molecular Sciences Laboratory which is part of the Pacific Northwest Laboratory, P.O. Box 999, Richland, Washington 99352, USA, and funded by the U.S. Department of Energy. The Pacific Northwest Laboratory is a multi-program laboratory operated by Battelle Memorial Institute for the U.S. Department of Energy under contract DE-AC06-76RLO 1830. Contact David Feller or Karen Schuchardt for further information